Ionic Lab Test
It should be noted that our Colloidal Silver is 70% ionic and the balance is Partical. So, no matter who you think is right, the water contains enough of each form of silver to be effective. We believe the ionic form of silver is the best form of Silver.

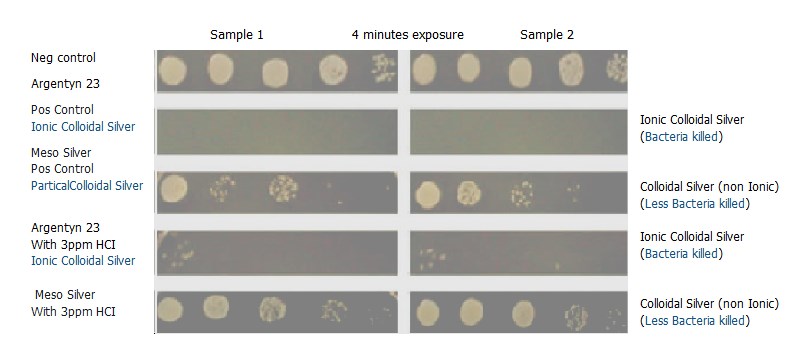
Test Report for Ionic Colloidal Silver
Results clearly show Ionic Silver is more effective.
The concentrations of HCl added to each of the two products were such that the concentrations of HCl in the final volumes would be 3ppm, 4ppm, and 7ppm. The range of concentration was determined in an earlier experiment. These concentrations were selected because, if the HCl were to react with the silver, the conversion of Ag+ to AgCl would not make HCl the limiting reagent. The amount of remaining active silver would be the true limiting factor.
1. It is evident from the results that the antimicrobial efficacy of both products decreases as the concentration of HCl increases.
2. When comparing the plates where no HCl was added, it can be seen that Argentyn 23 has far greater antibacterial efficacy than Mesosilver. Mesosilver’s antibacterial efficacy is almost negated by adding just 4ppm of HCl, whereas Argentyn23’s antibacterial efficacy is only slightly reduced but still strong, even with the addition of 7 PPM HCl.
3. Adding HCl to the silver products causes the chloride ion to bind the silver ion forming Silver chloride (AgCl). Since Argentyn23™ is primarily an ionic product there is still a sufficient number of Ag+ ions left to kill the bacteria.
4. Mesosilver, on the other hand, is primarily particulate in nature and has significantly little (compared to Argentyn 23) silver ion content. Once the silver ions in Mesosilver react with the chloride ions, they become almost completely inactivated. We see an almost complete loss of antimicrobial activity when Mesosilver is exposed to even modest quantities of HCl.
5. This experiment serves as proof that it is the active, available silver ions – not the particulate form – in which the antibacterial property of silver resides